PectaSol-C Modified Citrus Pectin Powder - 150 GRAMS
$40.00 To: Purchase
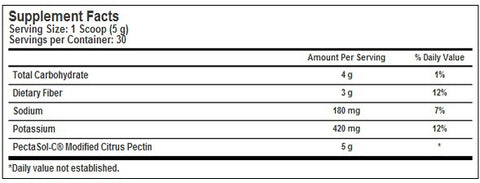
Dietary supplement. Promotes good health. Regular pectin, a soluble dietary fiber naturally present in citrus fruit, cannot be absorbed by the human digestive tract. However, PectaSol-C employs an enzymatic modification process (non-GMO) which creates smaller polysaccharide fragments that can be easily absorbed and utilized in the body. EcoNugenics PectaSol-C is made up of short chains of the proper molecular weight verified in recent scientific studies. The constituents of Modified Citrus Pectin that are believed to be responsible for its beneficial properties are the galactosyl fractions. Their toxin-binding properties in the bloodstream may help promote good health. (These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.) The natural color of the powder may have slight variations. Suitable for vegetarians and contains no dairy, egg, gluten, corn, soy or wheat. Contains no preservatives or artificial color, flavor or fragrance.
http://www.alsearsmd.com/natural-chelation/
Research from my colleague, Dr. Nan Fuchs, produced a remarkably safe and effective detoxifier.
It’s called modified citrus pectin, and it’s made from the inner peel of citrus fruits. Modified citrus pectin is one of the most powerful chelating substances I’ve found in the world, and it’s been proven to work in human clinical studies.
In one recent study, USDA scientists gave people modified citrus pectin for six days.4 The scientists measured the amount of toxins excreted in their urine before taking the modified citrus pectin and 24 hours after taking the citrus pectin.
The results?
- The amount of deadly arsenic excreted in participants’ urine increased by 130%…
- The amount of toxic mercury excreted increased by 150%…
- The amount of cadmium excreted increased by a whopping 230%…
- And the amount of toxic lead excreted increased by 560%.
Results like these in only 24 hours are remarkable.
And here’s something else: Even though modified citrus pectin is strong enough to soak up toxins and heavy metals like a sponge, it’s also gentle on your system. Not a single person in these human trials reported any side effects.
In fact, modified citrus pectin is so safe it has been tested on children as young as 5 years old who suffer from lead poisoning. Every child tested had a dramatic increase in the excretion of lead in their urine – over 130% on average.5
Modified citrus pectin is safer than many forms of chelation therapy, because it does NOT deplete the essential minerals your body needs. So while it eliminates toxic metals and pesticides like lead, cadmium, and mercury, it doesn’t deplete your body of zinc, calcium, or magnesium. In fact, there was no change in the level of these vital minerals in patients during the study.
_______________________________________________________
New Modified Citrus Pectin Slows Cancer Progression and Stops Cancer Cells in Their Tracks
Study Shows New Modified Citrus Pectin Works as Well as Chemo Without Toxicity and
Improves Quality of Life in Advanced Disease
SANTA ROSA, Calif.--(Business Wire)-- Better Health Publishing announced today that
many people with cancer could be helped by a new form of Modified Citrus Pectin
(MCP)that may work as well as chemotherapy in advanced disease stages; yet without
the dangerous toxicity. These published findings of new capabilities for MCP have the
potential to revolutionize integrative cancer healthcare.
Previous research with MCP has shown it to be effective in slowing the progression,
metastasis and angiogenesis of several types of cancer. Now with further improvement
in the modification process, more of the effective substance can be utilized by the body.
The first human study described below was done with patients with late stage, breast,
prostate, colorectal, kidney, pleural or lung, cervix/uterine cancer, liver, pharynx,
pancreatic, stomach, melanoma and bile duct cancers.
This landmark clinical trial was published in the peer-reviewed journal Clinical Medicine:
Oncology, where it showed a significant improvement of quality of life and stabilization
of disease for patients with advanced solid tumors. The results of the landmark
advanced tumor study clearly demonstrate MCP's enhanced capabilities.
"This is a very important breakthrough destined to help a lot of people," said Dr. Isaac
Eliaz, a prominent doctor of Integrative Medicine and MCP researcher. "MCP may have
the benefits similar to chemotherapy in advanced cancers, but without the side effects.
This study sheds additional light on the potential benefits of MCP in cancer prevention
and treatment."
A background report on the development of MCP in integrative cancer healthcare is
available through Better Health Publishing at the following url:
www.betterhealthpublishing.org/mcpreport
MCP, a natural substance modified from the peel of citrus fruit has been used during the
past decade in integrative cancer therapy tom help keep aberrant cells from clustering
and blocking the formation of blood vessel attachment. The absorption into the blood
stream is based on the size and weight of its molecules.
MCP molecules bind to receptors on cancerous cells, preventing these cells from
attaching to healthy tissue and blocking the formation of blood vessels to feed the
cancerous cells. Once this has occurred, the cancer cells starve and die or are
eliminated by the immune system.
In the past, the enzymatic modification process produced too much total breakdown of
the pectin molecules when it tried to achieve the effective low molecular weight
absorbable chains. The new MCP also goes through an enzymatic process that reduces
the pectin's molecular size and modifies it to better control the amount of total
breakdown of the citrus pectin. These new developments led to an improved MCP,
lower in weight, which allows it to be more systemic, circulating through the blood
stream and allowing the body to absorb more of the effective molecules.
Treatment and Scientific Data of Study:
The treatment in the advanced tumor and previous studies consisted of the oral intake
of five grams of MCP three times a day. One cycle of therapy was defined as four
weeks of treatment. Objectives were clinical benefit (pain, functional performance,
weight change), safety, tumor response (RESIST criteria) and quality of life.
The results proved very exciting. Forty-nine patients (between 36 and 82 years old) with
various advanced solid tumors were enrolled. Twenty-nine patients were evaluated for
clinical benefit after two cycles of treatment. All patients tolerated the therapy well
without any adverse effects. The results also demonstrated that after two cycles of oral
intake of MCP, 6/29, (20.7%) had an overall clinical benefit with a stabilization or
improvement of life quality. On intent to treat basis 11/49 patients (22.5%) showed a
stable disease after two cycles and 12.3% had a SD for more than 24 weeks. One
patient with advanced and hormonal resistant prostate cancer had a 50% decrease in
PSA with significant improvement in his quality of life. Most of the patients had
improvements in their life quality as reflected in the Karnofsky scores.
The MCP used in the advanced tumor study is now commercially available in the US.
It's called PectaSol-C and distributed by EcoNugenics, Santa Rosa, CA.
WARNING: MCP is profoundly constipating. Because of that we recommend that you
also take Homozon, which is a stool softener. Do not take at the same time. You can
take on same day. Taking Homozon 1/2 hour after MCP helps keep the bowel moving
while increasing the bodies ability to eliminate cancers. Also drink a good 12 ounces or
more of water with the MCP.